How is your fingernail sample prepared and analyzed?
- About 1 milligram of your fingernail clippings will be put
into a tiny tin foil capule, which will then be crushed closed.
- The tin capsules are loaded into an automated sampler which
will drop the samples, one by one, in to a furnace heated to
1100º C where the sample is instantly combusted to CO2,
N2, H2O, and other gases.
- A flow of helium then carries the gases through scrubbers
that remove unwanted gases, and a gas chromatograph column that
separates the remaining CO2 and N2 gases
so that they can be separately analyzed (Figure 2).
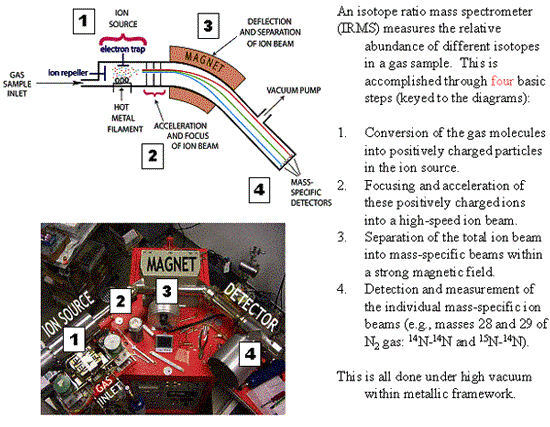
- The gases then flow into the mass spectrometer where the isotope
ratios of CO2 (13C/12C) and
N2 (15N/14N) are measured.
- A computer controls the mass spectrometer, collects the data,
and calculates the isotope ratios from the small voltages measured.
Fig. 2. Understanding how a mass spectrometer works.
View larger image
|
|

